Monitoring of waste degradation processes for sustainable MSW landfills
Contents [show]
Introduction
Across the world, the majority of MSW is landfilled, incinerated, placed in an open dump, or discarded into rivers or streets. Some cities have made enormous strides towards the ambitious goal of “zero waste”, particularly San Francisco, which has one of the highest recycle and reuse rates at 55% (Global Waste 2013). However, the reality remains that more than half of the U.S.'s MSW goes to landfills, and that this rate is similar or higher across the world. For the next few decades, while we work towards a “zero waste” economy, it is essential that we create landfill facilities that provide the highest environmental and economic benefits, as well as the lowest risk to contributing to climate change. Landfill facilities should strive to be sustainable - in essence leaving the world in equal or better shape to following generations. Next-generation and retrofitted bioreactor facilities appear to show great promise for providing these sustainable benefits.
Sustainable development requires MSW management that promotes public health, protects the environment, and is economically feasible. This is particularly true for low and middle-income countries that currently struggle to provide consistent waste management services. In Mumbai, open burning of waste is estimated to emit 10,000 grams toxin equivalents of carcinogenic dioxins/furans every year, causing 20% of the city’s air pollution (Annepu 2015). In Nepal, “garbage is…piled high in empty lots, on the roadside and on the edges of the city’s sewage-filled rivers…[with] [a]crid smoke from burning plastic filled the air.” (Lorch 2015). Increasing urban populations, changing consumption habits, natural disasters, and human conflicts weaken waste management services and threaten public health. The U.S. has been a pioneer in both innovative sustainable technologies and in MSW management practices. Experimenting with innovative technologies is risky and resource-intense; however, the rewards can be enormous for addressing our energy and environmental challenges, both domestically and internationally. With greater understanding of waste degradation processes landfill facilities can transform MSW from a hazard to be contained to a renewable energy source in the form of collected methane.
Recent studies on U.S. municipal solid waste (MSW) management have indicated that the U.S. is sending significantly more waste to landfills than previously estimated. One study published in 2015, indicated that the actual MSW disposed of in landfills is double that of current EPA estimates. For example, in 2012, the study finds that Americans disposed of 262 million metric tons of waste in landfills; while the EPA estimated 122 million metric tons (Powell et al. 2015). These findings are significant because MSW landfills, in their current state, represent a significant threat to the environment and climate change.
Modern MSW landfills were developed in response to the Resource Conservation and Recovery Act (RCRA) in 1976. For management of MSW, RCRA created Subtitle D landfills that are designed to minimize moisture addition to the landfill. Subtitle D landfills are also called sanitary or “dry-tomb” landfills, and operate to reduce the risk of leachate and gas emissions to the environment. Landfills emit methane in the form of biogas, which consists primarily of carbon dioxide and methane. Landfills represent the second largest anthropogenic source of methane in the U.S. at 18%; a significant contribution as methane’s impact on atmospheric pollution is considered to be 25 times greater than carbon dioxide over a 100-year period. Of 1754 landfills in the U.S., 558 (32%) have landfill gas collection systems that capture a portion of the biogas generated from landfills (EPA 2006). The gas collection systems operate sub-optimally, allowing anywhere from 50% to 90% of methane generated to be released into the atmosphere (Xunchang et al. 2015). In Subtitle D landfills the waste degrades slowly on the order of decades to centuries, however, the waste is isolated by containment systems with a significantly shorter design life.
The U.S. has experimented with improving MSW landfills through bioreactor landfills, which operate fundamentally as the opposite of Subtitle D landfills, with the encouragement of moisture addition to the waste. Bioreactor landfills recirculate either air or water to accelerate waste degradation and waste stabilization. Advantages of bioreactor landfills include accelerated waste degradation and stabilization in a matter of years rather than decades to centuries in “dry-tombs”, increased generation of biogas, lower waste toxicity, reduced leachate disposal costs, an estimated 15 to 30 percent gain in landfill space due to increased waste density, and reduced post-closure care. Despite these advantages, fewer than 2% of U.S. landfills are operated as bioreactors due to technological and scientific uncertainties (EPA 2006).
Bioreactor landfills lack robust data sets to demonstrate their benefits (Benson et al. 2006). Laboratory experiments and some field-scale experiments have demonstrated the above-mentioned advantages. Laboratory-scale experiments provide greater control and greater ability to measure parameters to describe the process of waste degradation. Waste degradation involves three interdependent physical processes, namely a fluid model of leachate and gas flows in the waste mass, a mechanical model of waste geotechnical properties, and a biochemical model describing aerobic and anaerobic degradation (Reddy et al. 2015). The field-scale demonstrations lack this control found in the laboratory, making it more challenging to obtain data that is indicative of the waste degradation process.
To address this challenge, researchers have explored the use of sensors and instrumentation at bioreactor landfills. These technologies provide a means to measure important parameters to describe the waste degradation processes. A better understanding of these processes can lead to optimization of bioreactor landfills to reach performance levels achieved in the laboratory. This paper will focus on sensors and instrumentation for leachate monitoring, gas monitoring, and in-situ monitoring of waste degradation processes.
Leachate Monitoring
Leachate Properties
Leachate is defined as liquid that has come into contact with waste. In a conventional landfill, leachate trickles down through the waste mass and into the leachate collection and removal system (LCRS) at the base of the landfill. In a bioreactor landfill, pipes are installed throughout the landfill to recirculate the leachate as evenly as possible throughout the waste. Leachate can be distributed horizontally or vertically, but the optimal method of even distribution depends on the characteristics of the specific landfill.
Physical and chemical leachate properties should routinely recorded as they help determine the effectiveness of the bioreactor. Some of the parameters that are commonly monitored in the leachate include:
- Chemical oxygen demand (COD) (mg/L)
- Biochemical oxygen demand (BOD) (mg/L)
- Dissolved oxygen content (mg/L)
- pH
- Temperature
- Redox potential (mV)
- Ammonia, nitrate/nitrite, phosphorus levels (mg/L)
- Heavy metals (chromium, nickel, zinc, cadmium, copper, lead, iron) (μg/L)
- Total dissolved solids (TDS) (mg/L or ppm)
The levels of these various parameters over time indicate how long the leachate should remain in circulation before being pumped out as well as how much treatment, if any, must be provided to the leachate before leaving the landfill site. The key parameters to measure stabilization progress are COD, BOD, level of nitrogen forms, and pH. Other parameters on this list are used to measure the amount of heavy metal sequestration brought about by recirculation. (MPCA, 2009)
BOD & COD
COD is the capacity of a body of water to consume oxygen during oxidation of inorganic chemicals (such as ammonia) and the decomposition of organic material. In contrast, BOD measures aerobic microorganisms' oxygen demand by microbial oxidation. The COD, in the case of landfills, is typically higher than the BOD, as shown in Figure 1. The higher the COD and BOD, the more the leachate is oxygen-deficient. In the initial stages after waste is deposited in a landfill, the BOD and COD quickly rise as the oxygen and nitrogen is depleted. As the organic matter is oxidized into methane and CO2 gases, the BOD and COD fall and reach a stable level. In a bioreactor landfill, stable levels are reached much faster than a conventional landfill. The lower the final BOD and COD, the less leachate treatment for organic matter is required upon leaving the landfill. However, with leachate recirculation, a potential issue that arises is large concentrations of heavy metals in the leachate. Extra treatment for heavy metal removal may need to be added post-circulation.
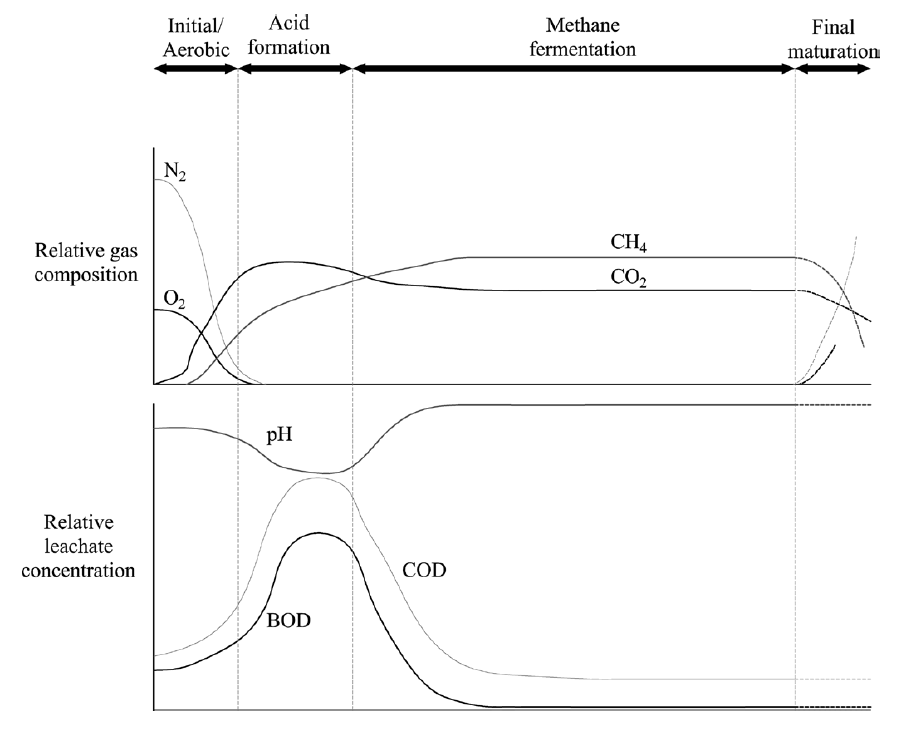
Figure 1: The process of waste stabilization over time during the aerobic and anaerobic phases of waste degradation. There is no scale on the x-axis; the amount of time it takes to reach the final mature stage is dependent on the specific parameters of the landfill. (Townsend et al. 2015)
Heavy Metals in Aerobic & Anaerobic Landfills
Heavy metal concentrations can be significantly different in an aerobic vs. anaerobic bioreactor. Landfill leachate typically contains high concentrations of heavy metals such as chromium, zinc, cadmium, copper, lead, and iron. Metals are much more likely to remain in the body of waste in an anaerobic bioreactor by sorbing onto soil particles and precipitating. In an aerobic bioreactor, the redox potential is reversed from strongly negative to positive, and metals are more likely to remain in the leachate as it exits the landfill. (Giannis et al. 2008)
Most aerobic reactors use a dual process: first acting as an aerobic reactor to accelerate the biodegradation of organic matter and then encouraging quicker methanogenesis during an anaerobic stage. Another added benefit of the dual bioreactor is more destruction of volatile organic compounds in the waste. (Waste Management Inc, 2015) Differences in leachate distribution systems between aerobic and dual systems is shown below.
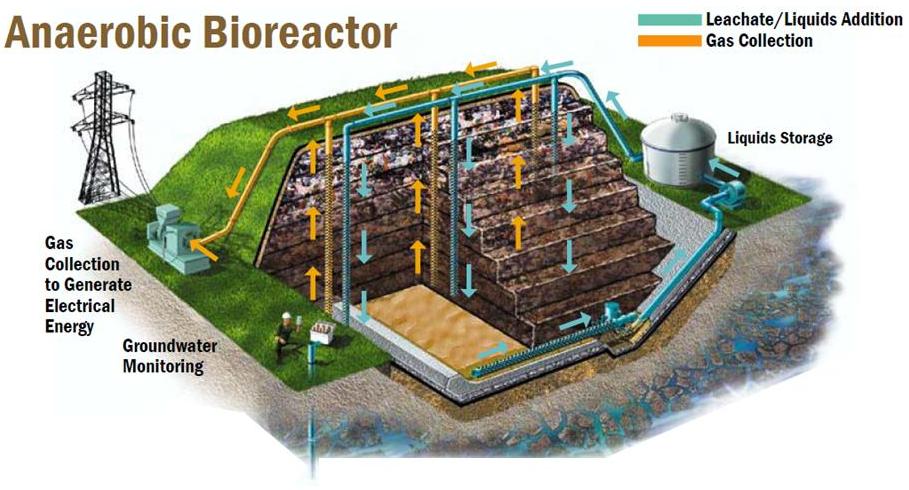
Figure 2: Process diagram for an anaerobic bioreactor with vertical leachate distribution and gas collection. (Waste Management Inc, 2012)
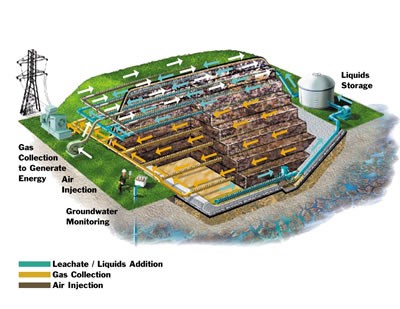
Figure 3: Process diagram for a dual aerobic-anaerobic bioreactor with horizontal leachate distribution, gas collection, and air injection. (Stauffer, 2006)
Monitoring BOD, COD, Heavy Metals, TDS
Tracking of leachate parameters can take place either in-situ within the landfill or within a leachate tank outside of the landfill. There has not been substantial research on leachate monitoring via sensors in the landfill; most of the current data on leachate parameters has been compiled from tests in recirculation tanks (see Figure 4). Each parameter is tested using methods described in the American Public Health Association’s (APHA) 1989 Standard Methods for the Examination of Water and Wastewater (SMWW).
One method to measure BOD and COD is using SMWW’s oxygen-consumption rate test. First, an oxygen-consumption rate device, such as a probe with an oxygen-sensitive electrode, is calibrated at a temperature similar to measured temperatures in the landfill. Then, the dissolved oxygen (DO) content of a leachate sample is increased by shaking the sample in a partially filled bottle or bubbling oxygen through it. The sample is added to a BOD bottle along with a biological suspension and a magnetic stirring bar, and the DO level is measured with the oxygen-sensitive probe and recorded. (APHA, 1989) The difference in the DO level over a period of five days represents the BOD5 value (a standard measurement of BOD).
Heavy metals in landfills often come from industrial waste, incineration waste, mine ash, batteries, paints, inks, and other hazardous household products. In a 2007 study in Greece, it was determined that the amount of heavy metals in the leachate vs. the amount remaining sorbed in the landfill was highly pH-dependent (Giannis et al. 2007). One of several ways to measure heavy metal concentrations is using ASTM D3987-85 shake extraction of solid waste with water procedure. A shaking device agitates leachate samples, and the samples are left to settle. The aqueous phase and solid metals are then separated by centrifugation, decantation, or filtration through a coarse paper. The remaining liquid is pressure filtered through a 0.45 μm filter paper. Different heavy metals can then be analyzed in the solid extract. (ASTM, 2004)
To determine the TDS of the leachate, standard methods consist of filtration to remove suspended solids, drying, and then measurement of the remaining mass. The bulk of TDS is made up of dissolved inorganic ions and organic matter. The inorganic ion (Cl-, SO42-, HCO3-, Na+, K+, Ca2+, Mg2+, etc) concentrations are measured using ion chromatography. The dissolved ion concentrations can provide information on the strength of the leachate; the concentrations generally increase over time as the leachate becomes less diluted with repeated circulation. (Townsend et al. 2015) The pH and temperature of the leachate can be measured either in the recirculation tank or within the landfill with simple pH and temperature meters, probes, and calibration fluids.

Figure 4: Diagram of leachate circulation and leachate control tank in a lab-scale bioreactor cell. (Abdallah, Kennedy, 2013)
Leachate Recirculation Considerations
Leachate is either immediately sent off to a wastewater treatment plant or receives partial on-site treatment before getting transported to a treatment plant. Some of the treatment can even occur in the leachate recirculation tank. The fraction of leachate that is re-circulated and the fraction that is removed from the waste mass for treatment should be carefully controlled. Some issues that may arise from over-circulation include ponding and overly acidic conditions. (Townsend et al. 2015). Overly acidic conditions disrupt the leachate treatment process, and pools of leachate within the landfill could be difficult and costly to remove.
The leachate can continually circulate through a section of the landfill even after the section is closed off to new waste until full stabilization in achieved. When the leachate COD is less than 1,000 mg/L and BOD is less than 100 mg/L, and the BOD:COD ratio is less than 0.1, and the gas production drops to 5% of its peak value, the waste is officially considered to be stabilized (Townsend et al. 2015).
Though it is difficult to achieve adequately even leachate circulation throughout the entire landfill, evenly spacing out leachate distribution pipes throughout ensures that the maximum possible amount of the waste mass comes in contact with the re-circulated leachate. At the same time, the pipes must be placed in such a way that lowers the risk of ponding and leaking. For example, in the state of Minnesota, leachate pipes must be at least 20 feet above the base of the waste and at least 50 feet from exterior slopes to minimize the risk of leachate seeps. (MPCA, 2009)
Leachate Blankets - Case Studies
Leachate is circulated through two conventional approaches: either vertical pipes or horizontal trenches, spaced out to minimize dry zones. A newer development in leachate distribution technology is the use of permeable circulation blankets. The high fluid conductivity blankets are composed of a nonwoven geotextile layer, two feet of shredded scrap tires, and another nonwoven geotextile on top. A variety of sensors are embedded in the blanket layer to monitor various parameters:
- Moisture content sensors
- Load sensor to monitor weight/stress of the waste
- Digital pressure gage to measure leachate head
- Magnetic flow meter
Sensor data is logged in an automatic data collection system. This technology was piloted at full scale in Jackson, MI in 2004, and the results indicated that the blankets were both hydraulically efficient for through leachate recirculation and cost-effective (Khire, 2004).


Figure 5: Testing permeable leachate circulation blankets in Jackson, MI. The blankets were created with shredded recycled tire scraps (left) and part of the automated sensor monitoring system is shown on the right.
In 1994, Yolo County, California began a field-scale test that compared anaerobic cells to aerobic cells in a landfill utilizing leachate recirculation. To distribute the leachate, horizontal trenches filled with shredded vehicle tires were installed throughout the landfill. The results indicated that the aerobic reactor allowed for greater organic decomposition than the anaerobic reactor because both aerobic and anaerobic reactions were able to take place throughout the waste mass. This was partially due to dead zones where flow did not reach, as well as "preferential flow pathways" where the tire-filled trenches were not able to evenly distribute the leachate (Townsend et al. 2015). Air was added to the aerobic section by using a vacuum system in the gas collection pipes to pull air in through a permeable soil cover. However, a major issue that arises from having a permeable cover is the seepage of water into the landfill during rainfall events, uncontrollably increasing the amount of circulated leachate.
Gas Monitoring Systems
The production of gas increases in bioreactor landfills as compared to conventional landfills along with the increased rate of waste degradation. A gas collection and control system (GCCS) is used to collect the primarily methane and CO2 gases that are produced as organic matter in the landfill is oxidized. The gas is either flared off, run through a passive biofilter system before entering the atmosphere, or used for energy generation. Because of the detrimental environmental effects of greenhouse gases, energy recovery is especially important in landfills with leachate recirculation and increased gas generation. To harness energy, the gas is first pressurized into pipes, water vapor removed in a condensing tank, compressed, chilled, reheated, and sent to a generator to generate electricity which is then distributed to the grid.

Figure 6: Typical landfill gas-to-energy process schematic. (EPA, 2013)
Important parameters to measure in the resulting gas include:
- Volume of total gas (ft3/min)
- Methane, CH4 - % by volume
- Carbon dioxide, CO2 - % by volume
- Oxygen, O2 - % by volume
- Other trace gases
- Pressure
- Temperature
The total gas production can be estimated using a mass flow meter, orifice plate, or pitot tube. (MPCA, 2009) A mass flow meter can only measure mass of gas per time, whereas orifice plates and pitot tubes can measure mass or volume of gas per time. The flow meters require the pressure and the temperature of the gas in order to calculate the flow rate. The pressure drop can be determined using a differential pressure transducer, and the temperature is measured using an in-line temperature gauge (Townsend et al. 2015). Mass flow meters also require the composition data of the gas before a flow rate can be determined. The composition of the gas at the exit site is measured using a landfill gas analyzer, see Figure 7 (Giannis et al. 2008).
The measurement of gas properties is important in determining the progression of waste decomposition; a graph similar to Figure 1 can be constructed for a particular landfill using carbon dioxide, methane, oxygen, and nitrogen concentrations over time. Gas composition data can also be used to predict the future gas production for a new batch of waste. The following formula is used to estimate gas generation:
G(t) = 2LokMoe-kt
where G(t) is the amount generated, Lo and k are the methane generation potential and rate constant, respectively (determined experimentally), and Mo is the mass of solid waste in the batch (Townsend et al. 2015). The coefficient of 2 assumes an approximately 50/50 CO2/CH4 composition; this value is adjusted based on the composition measured by the landfill gas analyzer.

Figure 7: Landfill gas analyzer diagram of a Nova system. The collected landfill gas passes into the analyzing machine and gas composition is reported. (Nova Gas, 2015)
Several times a year, in addition to monitoring the collected gas, the surface of all bioreactor landfills must be monitored for methane leaks as per the New Source Performance Standards (NSPS) 40 CFR 60. The “serpentine method” is carried out during dry meteorological conditions:
- A forward-looking infrared (FLIR) camera is used to detect potential leaks in landfill surface, especially the areas surrounding gas wellheads.
- A trained inspector carries a portable monitoring device. One option is a flame ionization detector (FID) portable gas analyzer.
- The inspector traverses the landfill by foot with the analyzer several inches above the ground surface.
- The upwind and downwind methane concentrations are measured.
- The inspector walks a serpentine path in order to cover the entire landfill surface, with the sampler continuously monitoring methane emissions.
- The path should not cross itself over the entire landfill surface. A separate range of concentrations is recorded for leachate recirculation areas and non-circulation areas (if applicable).
- Any methane concentrations greater than 500 ppm should be studied, repaired, and reevaluated according the NSPS standards.
In-situ Monitoring
To understand the complex physical and biochemical processes of waste degradation, moisture, temperature, and pressure must be measured in-situ at various depths. These parameters provide an indication of the activity phase of the waste degradation process. Measuring these parameters during waste degradation provides the necessary data to create models, and to work towards optimizing landfill operation. Since the 1970s, bioreactor landfills with leachate recirculation have demonstrated accelerated waste degradation accompanied with more rapid generation of methane. However, the processes for this accelerated waste degradation are poorly understood. In-situ monitoring enables operators and researchers to understand and optimize these processes to create more sustainable landfills.
Moisture content
Due to low moisture levels, MSW in sanitary landfills degrades at a very slow rate. Conversely, bioreactor landfills increase moisture levels to accelerate degradation. However, monitoring moisture at landfills presents a significant challenge due to preferential channeling of liquids in the waste. Studies have shown that liquids preferentially flow through a relatively small part of the waste, resulting in non-uniform waste degradation (Oonk et al. 2013). To be successful, bioreactor landfills must be designed and operated to have more uniform moisture levels through adequate recirculation of liquids. Moisture content sensors, at the current practice, are usually more indicative of relative moisture levels rather than of absolute moisture contents.

Figure 8: Preferential channeling of liquids in a MSW Landfill (Oonk et al. 2013)
Several methods have been proposed to measure moisture levels at landfills including borehole devices, buried instruments, and most recently surface techniques. Borehole devices include neutron probes which are lowered into boreholes and estimate the moisture content at varying depths of the surrounding waste. Neutron probes emit neutrons into the surrounding waste, and water results in the neutrons slowing down and a cloud building up around the probe. This neutron cloud is measured and calibrated, providing an estimate of moisture content (Townsend et al. 2015).
Ground instruments include time domain reflectrometry sensors (TDR), time domain transmissivity (TDT), and electrical resistance technology. TDR is conceptually similar to radar – the device emits an electromagnetic signal and then analyzes the reflected signal to measure physical characteristics of interest in the medium The reflected signal measured by TDR depends on the moisture content of the medium (typically soil), with moisture content based on the relative permittivity or dielectric constant of the material. TDT is similar, but the signal is analyzed but the emitted signal rather than reflected signal is measured. Both TDR and TDT are relatively expensive at approximately $500 per unit, electrical resistance devices on the other hand are approximately $25 per unit (Reinhart et al. 2002). Low cost moisture content sensors is essential because of the preferential flow previously mentioned, resulting in the extremely heterogeneous moisture levels within landfills.
Electrical resistance sensors are the most commonly used devices for moisture content measurement due to their low cost, their ease of installation, and their reliability. Electrical resistance sensors consist of a porous media with a pair of embedded electrodes. As moisture content in the porous media increase, the electrical resistance across the media decreases (Townsend et al. 2015). Typically, the porous media is a gypsum block or a granular matrix, and is insulated to prevent environmental conditions, such as salinity of the soil, from distorting measurements of the sensor (Reinhart et al. 2002).
Additionally, studies have attempted surface techniques for measurement of moisture contents at various depths through electrical resistivity tomography (ERT) (Reddy et al. 2015). ERT measures resistivity with depth, and shows general trends for moisture content; however, several limitations exist for this method. Resistivity measurements have limited resolution and the resolution decreases with depth, providing an average resistance for large sections of waste. Additionally, metal objects within the waste itself can distort the resistivity measurements (Oonk et al. 2013).
Temperature
Microbial activity is responsible for the biodegradation of waste and is an exothermic reaction. Consequently, while waste is undergoing degradation, landfills have significantly high internal temperatures compared to the outside air. Several studies have demonstrated temperatures as greater than 170° F (Townsend et al 2015). Monitoring the temperature in MSW at various depths provide an indication of the activity phase of the biodegradation process. Moreover, in-situ temperature measurement indicates when waste stabilization has been achieved (Lopes and Gomes 2013).
MSW landfills typically use thermistors and thermocouples to measure temperature in-situ at landfills. Both thermistors and thermocouples need to be designed to a range of expected temperatures, typically from -76 to 212°F (Townshend et al 2015). Thermistors and thermocouples can either be placed in the landfill as it is filled with MSW, or boreholes can be drilled after the MSW has been placed. Thermistors measure temperature by monitoring the variable resistance to temperature in a resistor. On the other hand, thermocouples consist of two wire strands that are made of different metals and then welded together at one end, at the junction of the wires, temperature is measured.
Additionally, techniques for measuring surface temperature can provide relative information on the level of waste degradation activity (Faisal 2011). Faisal found that land surface temperature (LST) for landfill sites was higher than surrounding areas, and weak correlation with LST and methane emission readings. Thermal imaging techniques are based on emissivity, which is defined as the fraction of energy being emitted relative to that of a black body. A black body is defined as the perfect emitter of heat energy with an emissivity value of 1. For example, pure water has an emissivity of 0.96, soil saturated with soil is approximately 0.95, and dry soil is 0.92. Thermal infrared cameras detect the infrared energy and converts this energy into an electronic signal. This signal is then processed to produce a thermal image, and the thermal image post-processed for temperature calculations.
Pressure
Real-time monitoring of pressure is essential for safe operation of landfills, especially those with leachate recirculation systems (LRS). Leachate injection, especially in landfill side slopes, results in excess pore fluid pressures and consequently lower shear strength. The lower shear strength compromises the slope’s stability and can result in failures at landfills (Reddy et al. 2015). Internal pressures within a landfill consist of pore pressures and pressure from the overburden weight of waste through total earth pressure cells (TEPC). Total earth pressure cells (TEPC) consist of a pressure transducer connected to a flat plate containing fluid. More overburden weight on the transducer results in a higher pressure reading. (Reinhart et al. 2002). TEPC provides changes in overburden pressure as additional waste lifts are placed at a location.
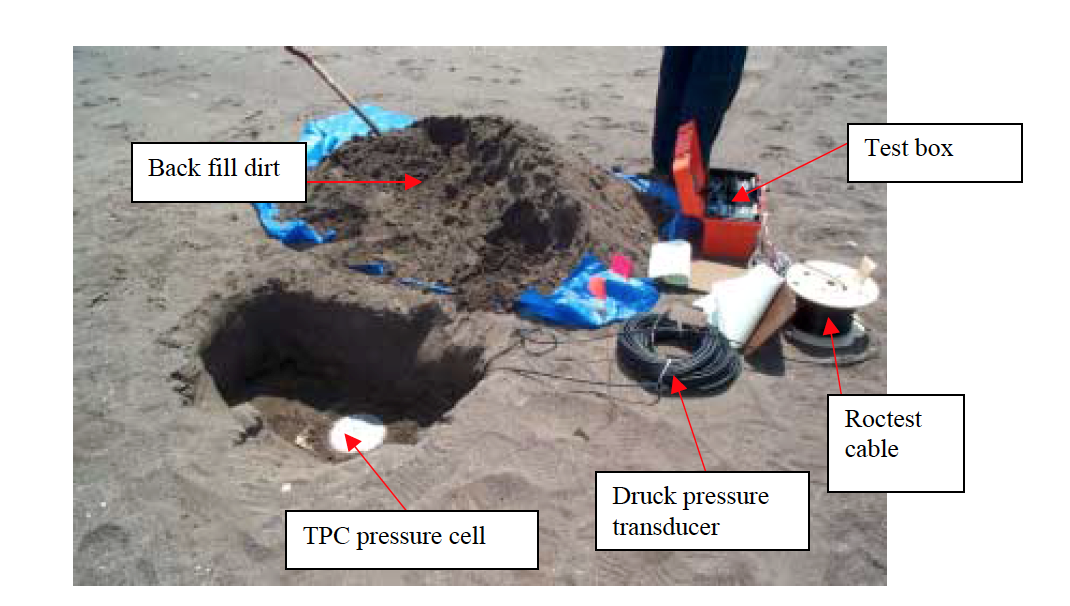
Figure 9: Installation of a total earth pressure cell (Reinhart et al. 2002)
Pore pressures are the combination of gas pressure and liquid pressure in the pore space of the MSW. Pressures are typically measured by buried electronic pressure transducers that are connected to a data logger and power source with a cable. Installation of pressure transducers and the accompanying cables is challenging in the landfill environment, and studies have proposed to development of wireless sensor networks to reduce the complexity and cost of monitoring (Nasipuri et al. 2006). Pressure transducers buried in waste have aided significantly in understand waste anisotropy, which results from the heterogeneous nature of landfills and results in non-uniform waste degradation (Townsend et al. 2015).

Figure 10: Installation of pressure transducers in borehole for in-situ pore pressure measurement (Townsend et al. 2015)
Conclusion
To address the shortcomings of “dry-tomb” landfills, the EPA and industry have experimented with bioreactor landfills. Advantages of bioreactor landfills include accelerated waste degradation and stabilization in a matter of years rather than decades to centuries in “dry-tombs”, increased generation of biogas, lower waste toxicity, reduced leachate disposal costs, an estimated 15 to 30 percent gain in landfill space due to increased waste density, and reduced post-closure care (EPA 2015). Despite these advantages, fewer than 2% of U.S. landfills are operated as bioreactors due to technological and scientific uncertainties (EPA 2015). Limited field data exist for landfills, though there is clear evidence that landfill gas collection systems are failing to efficiently capture gas resulting in methane release to the environment through cover soils that in turn lead to empiricism and inefficiencies in energy generation and collection (Pohland 1986). To optimize landfills and create the next-generation of sustainable landfills, processes must be actively and accurately monitorized to maximize energy collection, minimize environmental emissions, and use land as efficiently as possible to avoid encroachment on cities and communities.
The key components for monitoring landfills include leachate monitoring, gas monitoring, and in-situ monitoring. Leachate in conventional landfill slowly moves through the waste mass in an uncontrolled manner, where as in bioreactor landfills, it is recirculated as uniformly as possible to accelerate waste degradation. Key properties for leachate monitoring are chemical oxygen demand, biological oxygen demand, dissolved oxygen content, pH, temperature, redox potential, ammonia, heavy metals, and total dissolved solids.
Landfill biogas is generated during waste degradation, with the generation rate highly dependent on landfill operation and local climate. Gas collection and control systems (GCCS) are design to collect methane from biogas, in absence of GCCS, the methane is either flared or vented. Additionally, landfills are monitored for surface methane emissions to ensure that intermediate and final cover systems are adequate, and that the GCCS is operating as designed.
In-situ monitoring is necessary to understand the waste degradation processes, which typically occur over several years at significant depth. The three key parameters that distinguish conventional from bioreactor landfills for in-situ monitoring are moisture, temperature, and pressure (including both pore pressure and overburden pressure). Electrical resistance sensors is most commonly used for moisture content measures as it is low cost, reliable, and easily installed; however, its applications are limited to providing relative moisture contents in waste mass as opposed to absolute moisture contents. Temperature is measured by either thermistors or thermocouples, and must withstand extremely high temperatures up to 200°F, as research has demonstrated internal landfill temperatures of 170°F. Pressure is measured by pressure transducers buried in the landfill, with pore pressure representing both liquid pressure and gas pressure in surrounding pore space of waste and overburden pressure from the weight of waste lifts above the sensor.
Through monitoring of leachate, gas, and in-situ conditions, the waste degradation process can be better understood, and this knowledge applied to advancing the design and operation of MSW landfills. Field data collected by the sensors and instrumentation discussed can provide valuable information for incorporating the three physical interdependent processes that define waste degradation including a fluid model for leachate and gas, a mechanistic model of waste properties, and a biochemical model of the exothermic reactions during aerobic and anaerobic degradation. Through understanding and then control of degradation processes, MSW can be transformed from a hazard to be contained to sustainable energy source.
References
Annepu, R. (2015). "Tackling India's Waste Crisis." The Guardian. Web. 30 Apr. 2015. ." >http://www.theguardian.com/connect4climate-partner-zone/tackling-india-waste-crisis>.
EPA. (2015). "Bioreactors." Environmental Protection Agency, n.d. Web. 12 May 2015. ." >http://www.epa.gov/solidwaste/nonhaz/municipal/landfill/bioreactors.htm>.
Benson, C. H., Barlaz, M. A., Lane, D. T., & Rawe, J. M. (2007). Practice review of five bioreactor /recirculation landfills. Waste Management, 27(1), 13–29. http://doi.org/10.1016/j.wasman.2006.04.005
EPA. (2006). Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2006; Washington , DC 20460, 2007.
EPA. (2007). Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2006; Washington , DC 20460, 2007.
Global Waste on Pace to Triple by 2100." (2013). World Bank. Web. 12 May 2015. ." >http://www.worldbank.org/en/news/feature/2013/10/30/global-waste-on-pace-to-triple>.
Lopes, M.-L., & Gomes, C. C. (2015). Geotechnical landfill monitoring — adaptations needed. Environmental Geotechnics, 2(1), 8–17. http://doi.org/10.1680/envgeo.13.00014
Lorch, Donatella. (2015). "You Think Your City Is Full Of Trash? Ha!" NPR. NPR, 23 Mar. 2015. Web. 12 May 2015. ." >http://www.npr.org/sections/goatsandsoda/2015/03/23/394827432/you-think-your-city-is-full-of-trash-ha>.
Oonk, H., Zomeren, A. van, Rees-White, T. C., Beaven, R. P., Hoekstra, N., Luning, L., Woelders, H. (2013). Enhanced biodegradation at the Landgraaf bioreactor test-cell. Waste Management, 33(10), 2048–2060. http://doi.org/10.1016/j.wasman.2013.03.003
Powell, J. T., Townsend, T. G., & Zimmerman, J. B. (2015). Estimates of solid waste disposal rates and reduction targets for landfill gas emissions. Nature Climate Change. http://doi.org/10.1038/nclimate2804
Pohland, F. G.; Harper, S. R. (1986). Critical Review and Summary of Leachate and Gas Production from Landfills; Cincinnati, Ohio 45268.
Reddy, K. R., Giri, R. K., & Kulkarni, H. S. (2015). Modeling Coupled Hydromechanical Behavior of Landfilled Waste in Bioreactor Landfills: Numerical Formulation and Validation. Journal of Hazardous, Toxic, and Radioactive Waste, D4015004. http://doi.org/10.1061/(ASCE)HZ.2153-5515.0000289
Reinhart, D., Townsend, T., McCreanor, P. (2002). Florida Bioreactor Demonstration Project – Instrumentation. Waste Tech 2002 – Landfill Conference.
Townsend, T. G., Powell, J., Jain, P., Xu, Q., Tolaymat, T., & Reinhart, D. (2015). Sustainable Practices for Landfill Design and Operation. New York, NY: Springer New York. Retrieved from http://link.springer.com/10.1007/978-1-4939-2662-6
Xunchang, F., Zekkos, D., Raskin, L. (2015). Quantification of parameters influencing methane generation due to biodegradation of municipal solid waste in landfills and laboratory experiments. Waste Management, currently under review for publication.
 Julie Bateman
Julie Bateman  Sierra Schatz
Sierra Schatz 




























2 COMMENTS
Eugene Gallagher*
Nov, 30, 2015 Julie, SierraWell done on getting your assignment to this stage. There is a good body of work in what you have done so far; there was lots to cover and you have done a reasonable job of that.
Some quick comments - I would have liked to see your references in full - they were not visible in the web version. Also I would have liked to see some non-US experience or as a minimum recognition that there are alternative approaches and paradigms, not least regulatory perspectives, including different interpretations on risk-based decision making, especially in Europe.
Your title includes the word 'sustainable' in the context of MSW landfills, but you do not really define this concept or your understanding of it; further you do not really return to this theme in your conclusions. Engaging with this topic more would probably strengthen your assignment and 'close the circle' more. If you don't use the word it becomes an empty statement, an unfulfilled promise to your readers.
You do start to discuss landfill degradation processes from a systemic albeit limited viewpoint in para 4 (Intro), but I would suggest this approach of using various systems perspectives might be very fruitful if you widened it to include a number of levels:
how does a landfill operate as a system of interest within its own domain (the landfill, waste body and near field)? how does it operate/impact when considered from a local societal and environmental domain (the region from which waste is generated and the environmental setting potentially affected by it)? and
how does it operate as one element in a wider waste management cycle where landfilling is only one and not necessarily the optimal option?
Asking those wider questions might give you a better 'red thread' to run through your assignment, something you can use as a framework, to explore as you go through the elements of your submission and then return to in your conclusions.
Your conclusions are somewhat weak and the work tails off because of that.
I suggest you use the opportunities presented to you in developing your conclusions to reflect much more deeply on the 'why, not just the 'what' of landfill monitoring, and then repeatedly to ask yourselves "have we answered the 'so what' question": the deeper why.
Good luck with this, and with your future careers!
Julie Bateman
Dec, 05, 2015 Dear Eugene,Thanks very much for comments! Agreed on there being an enormous amount to cover on the subject on design and operation of sustainable landfills.
Thank you very much for notifying us of our significant oversight for not posting the references on the website. The references are now available beneath the Conclusion.
Our article is very U.S.-centric, and we hope in the future to study Europe's design of landfills and regulatory frameworks in greater detail as there is so much to learn. We were particularly impressed by Dr. Oonk's work in the Netherlands on a 10-year project with bioreactor. We did not explicitly state that this work was done in the Netherlands, and we are indebted to Dr. Oonk and his team for helping us better understand moisture gradients in landfills.
Thank you for the comments on the thread of sustainability and on strengthening our conclusions. We have adapted the introduction and conclusion to provide a framework for addressing the sustainability aspect of landfills, and its context in the wider world. We view the body of the work - leachate monitoring, gas monitoring, and in-situ monitoring - as necessary steps for understanding waste degradation to achieve more sustainable landfills.
Best,
Julie
Edit Comment